
PharmaShots Weekly Snapshots (November 11 – November 15, 2024)
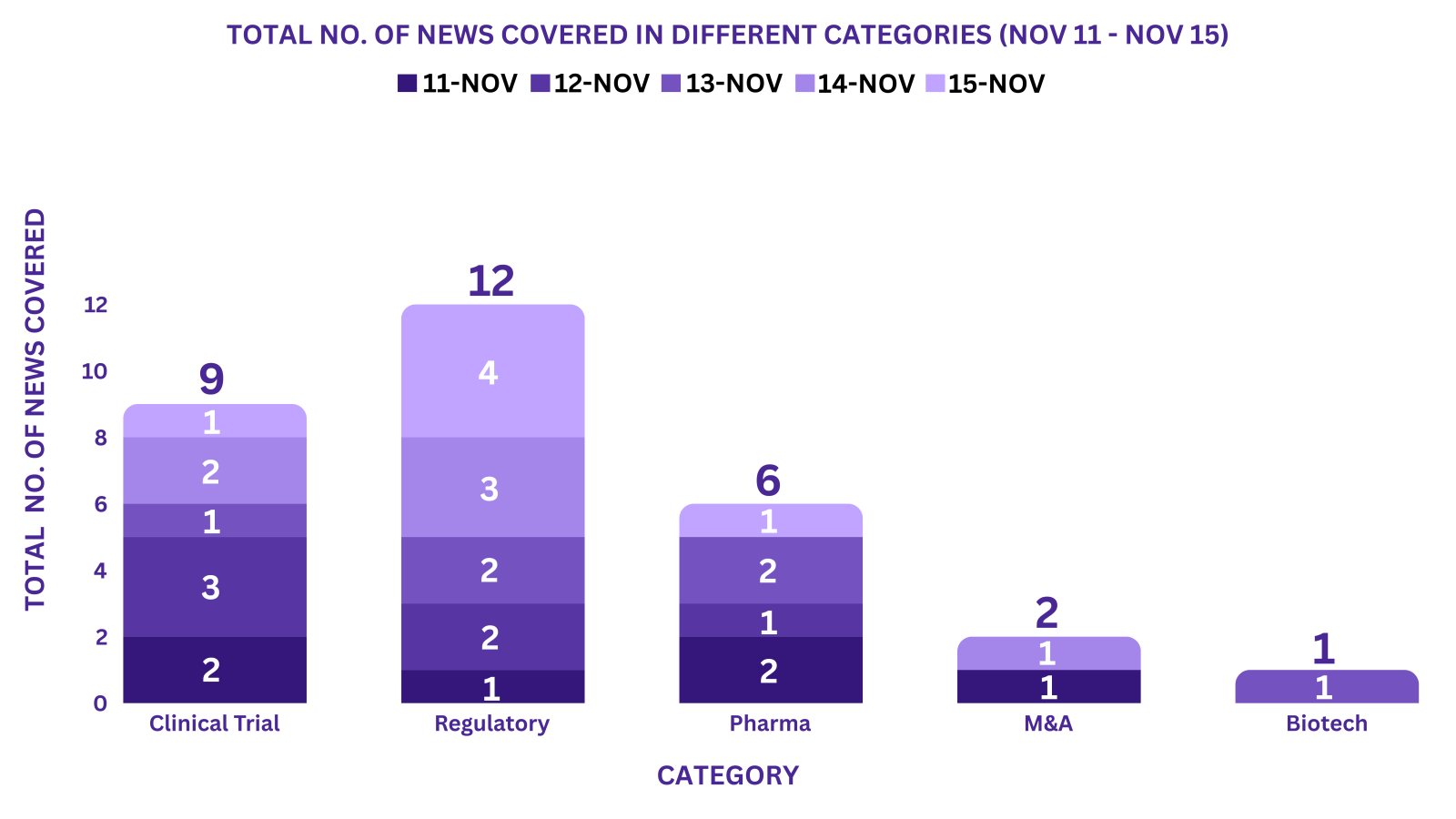
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, M&A, & Biotech. Check out our full report below:

Paratek Pharmaceuticals Reports Topline Results from P-IIb Study of Nuzyra (Omadacycline) for Nontuberculous Mycobacterial Abscessus Pulmonary Disease
Read More: Paratek Pharmaceuticals
Relief Therapeutics Reports Final Data from the Study of RLF-TD011 for Treating Epidermolysis Bullosa
Read More: Relief Therapeutics
AbbVie Reports the P-II (EMPOWER) Studies Data of Emraclidine for Treating Schizophrenia
Read More: AbbVie
Merck KGaA Reports Results from the P-III (MANEUVER) Study of Pimicotinib for Treating Tenosynovial Giant Cell Tumor (TGCT)
Read More: Merck KGaA
AstraZeneca Provides Update on P-III (KOMET) Study of Koselugo for Treating Neurofibromatosis Type 1
Read More: AstraZeneca
Syros Pharmaceuticals Reports the P-III (SELECT-MDS-1) Study Data of Tamibarotene for Treating Myelodysplastic Syndrome
Read More: Syros Pharmaceuticals
Structure Therapeutics Reports the First Patient Dosing in P-IIb (ACCESS) Trial of GSBR-1290 for Obesity
Read More: Structure Therapeutics
GSK Provides Update on P-III (DREAMM-7) Trial of Blenrep for Treating R/R Multiple Myeloma
Read More: GSK
Johnson & Johnson to Feature Analysis from P-II (DAHLIAS) Study of Nipocalimab for Treating Sjögren’s Disease at ACR Convergence 2024
Read More: Johnson & Johnson

Autolus Therapeutics’ Aucatzyl (Obecabtagene Autoleucel) Receives the UA FDA’s Approval to Treat R/R B-Cell Acute Lymphoblastic Leukemia
Read More: Autolus Therapeutics
Atamyo Therapeutics Reports the US FDA’s IND Clearance of ATA-200 for Treating Limb-Girdle Muscular Dystrophy Type 2C/R5 (LGMD2C/R5)
Read More: Atamyo Therapeutics
argenx and Zai Lab Report the NMPA’s Approval of Vyvgart Hytrulo for Chronic Inflammatory Demyelinating Polyneuropathy
Read More: argenx and Zai Lab
GSK’s Ojjaara (Momelotinib) Receives the Health Canada’s Approval to Treat Myelofibrosis in Adults with Moderate to Severe Anemia
Read More: GSK
Caliway Biopharmaceuticals’ CBL-514 Receives the EMA’s Orphan Drug Designation for the Treatment of Dercum’s Disease
Read More: Caliway Biopharmaceuticals
Genascence Corporation’s GNSC-001 Receives the US FDA’s Fast Track Designation for Treating Osteoarthritis (OA) of the Knee
Read More: Genascence Corporation
Actuate Therapeutics’ Elraglusib Receives the US FDA’s Rare Pediatric Disease Designation for Treating Ewing Sarcoma
Read More: Actuate Therapeutics
PTC Therapeutics Reports the US FDA’s Accelerated Approval of its Gene Therapy for AADC Deficiency
Read More: PTC Therapeutics
Eisai and Biogen Reports the CHMP’s Positive Opinion of Lecanemab to Treat Early Alzheimer’s Disease
Read More: Eisai and Biogen
Regeneron and Sanofi Report the US FDA’s sBLA Acceptance of Dupixent (Dupilumab) to Treat Chronic Spontaneous Urticaria (CSU)
Read More: Regeneron and Sanofi
AlveoGene’s AVG-0020 Secures the US FDA’s Rare Pediatric Disease Designation (RPDD) for Lethal Neonatal Surfactant Protein B (SP-B) Deficiency
Read More: AlveoGene
BMS’ Repotrectinib Gains the CHMP’s Positive Opinion to Treat Advanced ROS1+ NSCLC and NTRK+ Solid Tumors
Read More: BMS

Alteogen and Daiichi Sankyo Partner to Develop and Commercialize SC Formulation of Enhertu
Read More: Alteogen and Daiichi Sankyo
IDEAYA Biosciences Nominates IDE034 as a Development Candidate Under Partnership with Biocytogen
Read More: IDEAYA Biosciences and Biocytogen
Apollo Therapeutics Joins Forces with Sunshine Lake Pharma to Develop APL-18881 for Multiple Indications
Read More: Apollo Therapeutics and Sunshine Lake Pharma
Schrödinger Enters into a Multi-Target Collaboration and Expanded Software Licensing Agreement with Novartis
Read More: Schrödinger and Novartis
Nippon Shinyaku Collaborates with Atsena Therapeutics to Commercialize ATSN-101 Across the US and Japan
Read More: Nippon Shinyaku and Atsena Therapeutics
Merck Join Forces with LaNova Medicines to Advance LM-299 for Advanced Solid Tumors
Read More: Merck and LaNova Medicines

Kalaris Therapeutics Reverse Merges with AlloVir to Advance Therapies Focussing on Retinal Diseases
Read More: Kalaris Therapeutics and AlloVir
BioNTech to Acquire Biotheus, Enhancing its R&D Capabilities in Oncology and Expanding Reach Across China
Read More: BioNTech and Biotheus

Flare Therapeutics Inks Pact with Roche to Develop Small Molecules Targeting Undrugged Transcription Factors in Oncology
Read More: Flare Therapeutics and Roche
Related Post: PharmaShots Weekly Snapshots (November 04 – November 08, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














